Health Products and Technologies (HPT) - Pharmaceuticals
Health Products and Technologies (HTP) - The Pharmaceuticals domain encompasses a range of standardized concepts and terminologies related to the management, distribution, and use of pharmaceutical products. This domain is essential for ensuring that medicines and related health technologies are accurately described, tracked, and utilized within healthcare systems. It generally includes the following components:
-
Pharmaceutical Products: Standardized names, descriptions, and classifications for different drugs and medications, including generic and brand names. This could involve categorizing drugs by their active ingredients, therapeutic use, and formulations (e.g., tablets, injections).
-
Drug Classifications: Systems for categorizing pharmaceuticals based on their pharmacological properties, therapeutic use, or chemical structure.
-
Dosage Forms: Standardized concepts related to the different forms in which pharmaceuticals are available, such as tablets, capsules, liquids, injections, and topical applications.
-
Strengths and Concentrations: Definitions and standardized terms for the strength or concentration of active ingredients in pharmaceutical products, which is critical for accurate dosing and prescribing.
-
Regulatory Information: Standardized data elements for capturing regulatory information, such as drug approval status, licensing, and compliance with national or international standards.
-
Adverse Drug Reactions (ADRs): Terminologies and concepts for documenting and reporting adverse drug reactions, which are critical for pharmacovigilance and patient safety.
-
Prescription and Dispensing: Standardized concepts for prescribing and dispensing practices, including terms for prescription orders, dispensing quantities, and patient instructions..
-
Medication Management: Concepts related to the use and monitoring of medications, including patient adherence, medication reviews, and therapeutic drug monitoring.
These concepts are sources from the Pharmacy and Poisons Board of Kenya
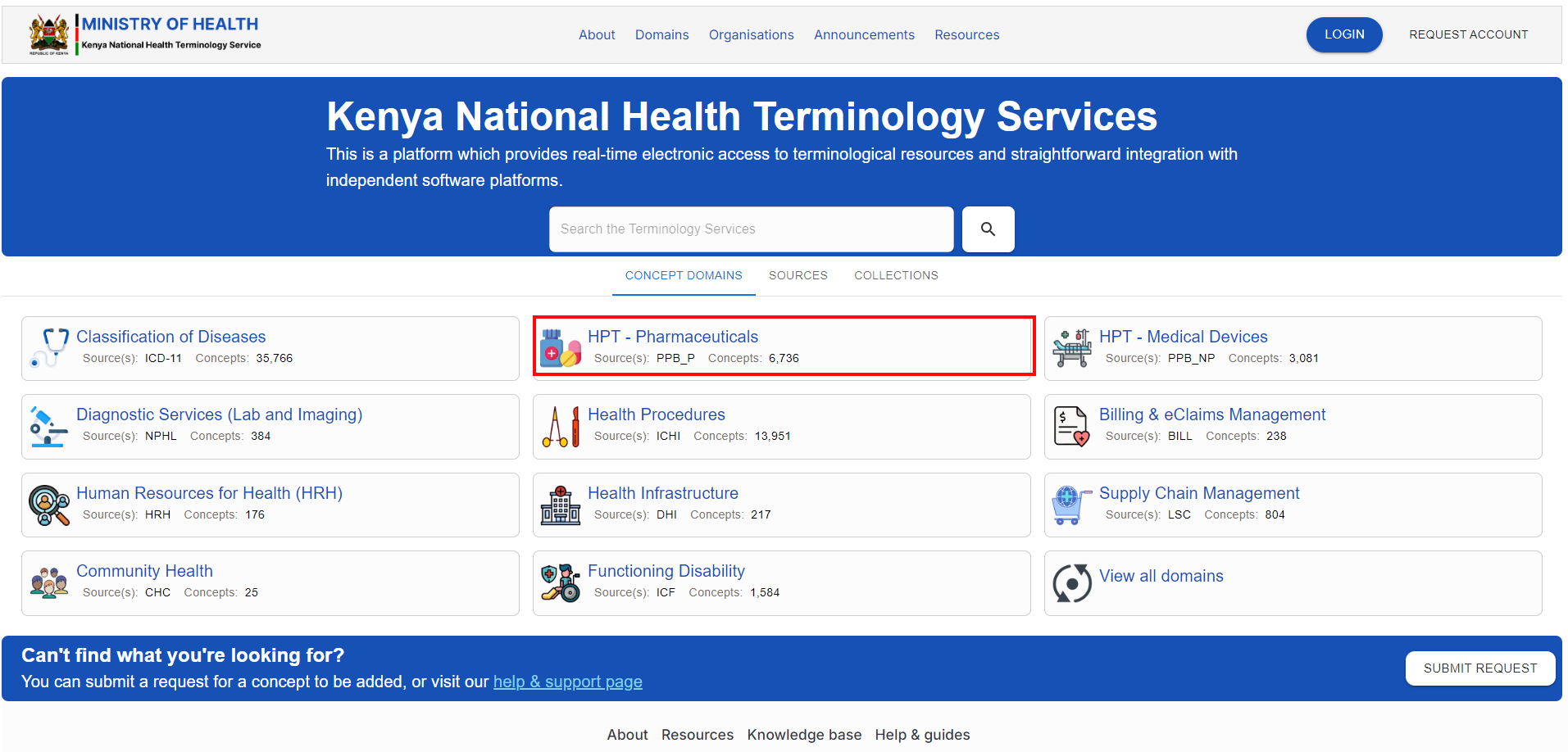
To access the domain, navigate to the KNHTS Homepage and select the highlighted domain below
Refer to the Classification of Diseases page in this user guide to see navigate around and perform actions related to concepts